Contents
- 1 Overview
- 2 Essure Birth Control Device
- 3 Side Effects and Complications of Essure
- 4 Is Essure Reversible or Removable?
- 5 Why are Women Filing Lawsuits on Essure?
- 6 FDA Actions and Adverse Events Timeline
- 7 Will The Birth Control Lawsuits be Stopped by Preemption Laws?
- 8 Essure Litigation in Process
- 9 Essure Multidistrict Litigation (MDL 2739)
- 10 Verdicts and Settlements in Essure Lawsuits
- 11 Status of Essure Litigation
- 12 Essure Cases that have been Dismissed
- 13 Filing an Essure Lawsuit
- 14 Time Limits for Filing a Claim
- 15 Get Legal Assistance – Contact an Essure Lawyer
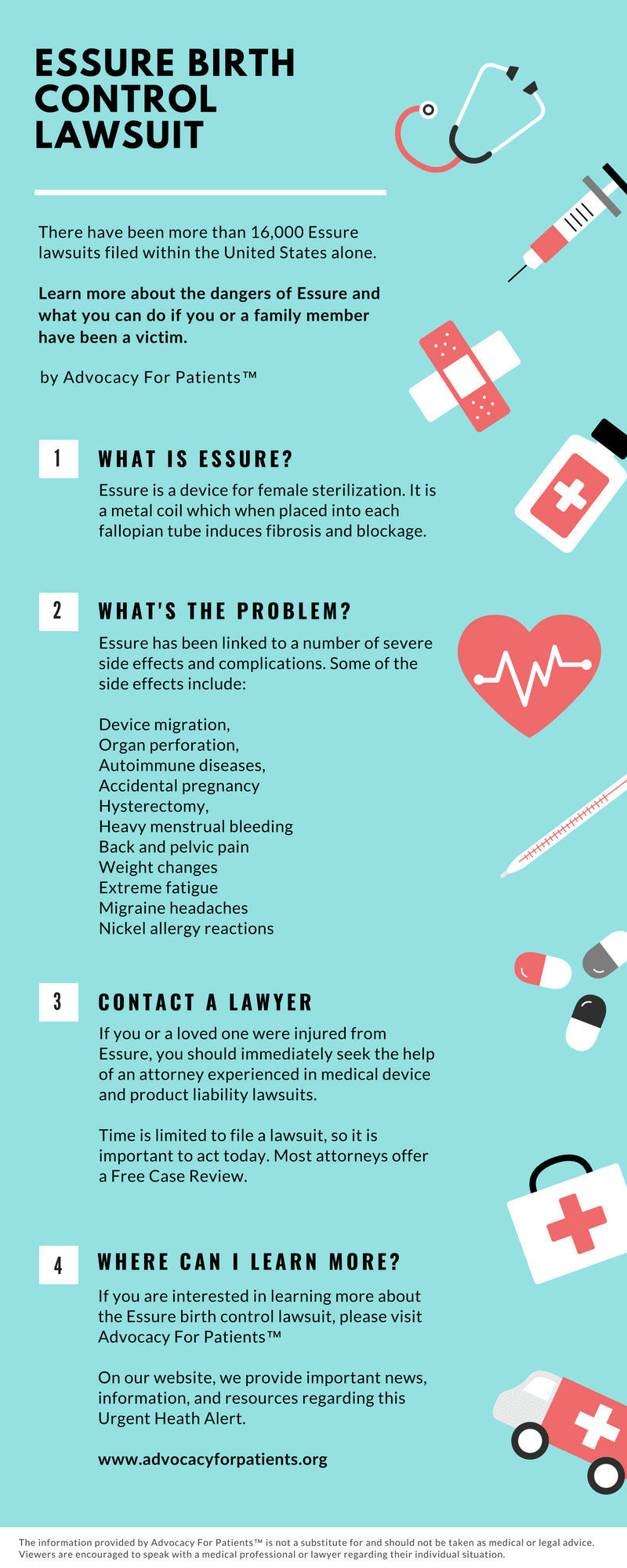
Update: On July 20th, 2018, Bayer said that it will discontinue the sales of Essure in the United States at the end of the year. Something that patient advocates and injury lawyers have been working towards for many years after thousands of women have experienced serious health complications.
If you are wondering how to join the Essure lawsuit, skip to the bottom of this page to fill out the contact form for a Free Case Review.
Overview
Birth control, or contraception, is the deliberate prevention of pregnancy. The Centers for Disease Control and Prevention (CDC) reports that 15.9% of women in the United States are currently using the pill and 8% are using a contraceptive implant or intrauterine device (long-acting reversible contraception).
From 2011 to 2013, of the 60.9 million women aged 15 to 44 in the US, 61.7% were using contraception. The most common methods of contraception being used were contraceptive pills (16%), female sterilization (15.5%), long-acting reversible contraceptives (7.2%), and male condoms (9.4%).
Essure Birth Control Device
Essure is a permanent birth control device that consists of two small metal coils that prevent eggs from moving through the fallopian tubes when it is implanted. It thus prevents fertilization and implantation. Although it does not involve “tying” the fallopian tubes, it is technically a form of tubal ligation.
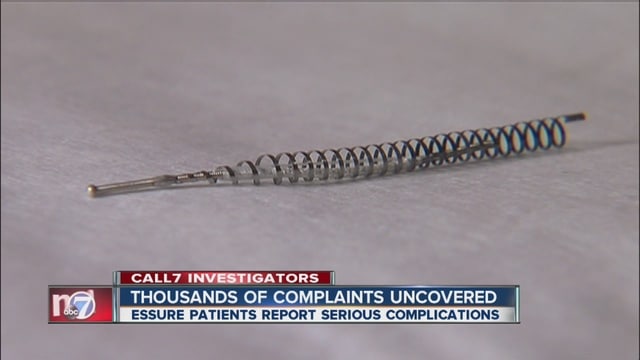
The contraceptive device is manufactured by Bayer Healthcare, the medical device-making giant. The company markets Essure as a “permanent contraceptive.”
Bayer claims that only a minimally invasive procedure is required for Essure devices and that the procedure can be performed right in a doctor’s office. However, women who receive the device must continue to use other birth control forms for a minimum for 3 months after insertion to confirm that it is effective and that it will prevent pregnancy.
Unfortunately, there are many people who have reported experiencing severe side effects and complications after implantation of Essure.
These issues include everything from allergic reactions and pelvic pain to needing additional surgeries for the removal of the device or repairing organs that have been damaged due to the birth control implant.
Side Effects and Complications of Essure
For many women, the side effects of the device started immediately after it was implanted. Some women have undergone several removal surgeries due to complications caused by Essure.
As of January 2018, there were 26,272 reports of adverse events for the device filed to the FDA.
Side effects mentioned:
- Allergies to nickel
- Bleeding
- Bloating
- Depression
- Heavy periods
- Severe pelvic pain
- Rashes or itching
- Hair loss
- Migraine headaches
- Menstruation problems
- Weight gain
- Back pain
- Brain fog
- Metal allergies
- Chronic fatigue
- Chronic or severe pain
- Scar tissue
- Unwanted or unintended pregnancy
- Ectopic pregnancy
- Autoimmune disorders
- Perforation of the colon, uterus or another organ
- Fetal death
- Breakage, migration or expulsion of the device
- Hysterectomy
- Additional surgeries
- Also death
Is Essure Reversible or Removable?
According to the label of the contraceptive device, the Essure procedure is a permanent one and irreversible. However, in cases where women have experienced side effects, the coils of the Essure device can be surgically removed.
Why are Women Filing Lawsuits on Essure?

As a result, in April 2018, the agency restricted sales of the device. Bayer Healthcare announced in July 2018 that it would discontinue the sale of Essure in the US by December 31st, 2018.
There are more than 16,000 lawsuits for Essure that have been filed against the manufacturer. Plaintiffs claim that Bayer did not warn patients of the potentially severe complications. Many women are filing lawsuits against the manufacturer because of the numerous complications they experienced after implantation of the company’s birth control device.
FDA Actions and Adverse Events Timeline

Bayer also had to submit any reports of adverse events, on a regular basis. Despite these requirements, the FDA cited the company a number of times for violations of those conditions, some of which resulted in the regulatory agency’s future actions:
June 2008: The FDA notified Bayer about a discovery it made about the company making Essure devices in an unlicensed factory at least since 2005, and that the medical device maker had failed to appropriately document their procedures.
January 2011: The FDA cited Bayer for using materials that were not approved in the Essure implant. This was a violation of the premarket approval, which required the manufacturer to seek additional approval for any changes in design.
February 2016: Bayer was ordered by the FDA to conduct a new clinical trial to determine whether Essure had any heightened risks for certain women, based on reports of adverse events that were filed to the regulatory agency.
November 2016: The FDA required the addition of a black box warning on the labels of the Essure device indicating the risks of allergy, abdominal pain, and the perforation, among other potential complications.
April 2018: The sales of Essure was restricted by the FDA to medical centers and doctors that promised to conduct a conversation and go through a checklist with the patient before they prescribed the birth control implant.
July 2018: The manufacturer of Bayer announced that they would discontinue selling the Essure birth control device by the end of the year, ultimately bowing to a lengthy campaign by health advocates and thousands of women to get the device off the market.
Will The Birth Control Lawsuits be Stopped by Preemption Laws?
Bayer Healthcare has made attempts to get a number of the Essure implant lawsuits dismissed as a result of certain laws that are known as preemption laws.
According to the company, it cannot be held liable for problems with the contraceptive device because the FDA conducts a rigorous series of tests on medical devices before approving them and deemed Essure to be generally safe.
There are a number of levels on which this argument is problematic, especially since it is clear that Bayer did not always follow its obligations on post-market regulations.
Nonetheless, using this argument, the company has succeeded in getting some product liability lawsuits dismissed while others have been allowed to move forward, with modified complaints in many cases.
If you have not contacted a lawyer to discuss your potential case, now is the time. Please do not wait, as you could be forever time-barred from pursuing an IUD lawsuit against the company.
Essure Litigation in Process
According to the most recent financial reports of Bayer Healthcare, approximately 16,100 lawsuits have been filed in state and federal courts across the US. At this time, all of these lawsuits are individual legal actions.
There are no current Essure class action lawsuits or any MDL (multidistrict litigation) processes that have been established. As of April 2018, a report from Bayer Healthcare stated that there were two lawsuits in Canada seeking class action certification.
Essure Multidistrict Litigation (MDL 2739)
A group of 28 plaintiffs had submitted a petition in 2016 to transfer their cases using the MDL process that was overseen by the Judicial Panel on Multidistrict Litigation. However, a short time after the petition was filed, a judge in the Eastern District of Pennsylvania – where 23 cases of the 28 had been filed – agreed to the consolidation of the cases in that court. The motion for a multidistrict litigation was withdrawn, resulting in the cases to remain separate.
In some cases, such as California, processes that use MDL system-like processes (called a JCCP) have been established to coordinate Essure cases. However, so far, there have been no efforts made so as to enforce a federal MDL.
There is a possibility of an MDL being created in the future due to the rise in the number of actions that are being filed.
Verdicts and Settlements in Essure Lawsuits
To this day, there have been no major publicized verdicts or settlements awarding large amounts of compensation to women who have been injured by the contraceptive devices. However, Bayer could decide to try to settle them due to a large number of lawsuits that are currently going through the court system.
This decision could come if it looks like the medical device maker is likely to lose cases based on the claims’ legal merits.
These suits are ongoing. Trials have not been set, so, as mentioned earlier, there are no jury verdicts or settlements. Lawsuits in California may be the first to go to trial. Jury verdicts from the first cases may have an influence on jury verdicts in other states.
In May 2018, both sides were ordered to name Special Settlement Masters by Judge John R. Padova in Pennsylvania in the event of a settlement. Special Settlement Masters is a firm that handles any potential settlement in lawsuits.
Potential compensation may result from damages that include:
- Medical bills
- Pain and suffering
- Lost time from work
- Loss of income
- Loss of quality of life
- Loss of companionship
- And more
Status of Essure Litigation
Women and their families continue to file claims in state courts across the United States. Most of the lawsuits are in Pennsylvania, California, North Carolina, and Missouri. In February and April 2018, the latest Essure lawsuits were filed by plaintiffs in Pennsylvania federal court.
Women who filed in Pennsylvania said that the device migrates from the fallopian tubes, breaks into pieces, perforates organs, and/or corrodes and wreaks havoc on the female body.
In March 2018, Judge Winifred Y. Smith of California made it mandatory for plaintiffs to submit a master complaint in which allegations against Bayer should be combined. She also stated that both parties should begin their preparations for trial. As of March 2018, Judge Smith presided over more than 13,000 California lawsuits on Essure.
Essure Cases that have been Dismissed
There have been some judges that have dismissed Essure cases for preemption. This puts a limit on product liability in state lawsuits after approval from the FDA. As mentioned earlier, Judge Smith permitted some of the lawsuits in California to proceed in spite of preemption.
Other cases have been dismissed for failing to meet the requirements of the court. For example, in January 2018, Judge Stephen Lambaugh of Missouri federal court threw 92 out of 95 plaintiffs from an Essure case.
IMPORTANT: Just because some cases have been dismissed does not mean that you are out of luck. Regardless of which state you live in, if you have been injured by Essure, you have legal rights and can still contact an Essure lawyer to discuss filing a case.
Filing an Essure Lawsuit

Most lawyers will conduct a review of your potential case for free.
Once the free case evaluation is done, your lawyer will let you know if they are interested in moving forward with your claim.
You will not have to pay an Essure lawyer for their services if they take on your case as they usually work on a contingency basis. This means that you will not pay anything unless there is a jury verdict or settlement to your benefit. When you obtain compensation from the lawsuit, your lawyer will receive a negotiated amount for fees and expenses.
Time Limits for Filing a Claim

Your lawyer will find out the time limit in your state and make sure that you file your case before that time limit. It is important to file your claim before the statute of limitations ends or you might lose your right to pursue future action.
If you haven’t already, you should contact an Essure injury lawyer immediately to discuss your defective medical device case.
Get Legal Assistance – Contact an Essure Lawyer
If you or a loved one has experienced side effects or suffered complications as a result of the Essure birth control device, you should immediately seek the help of a qualified lawyer.
To speak with a lawyer handling Essure lawsuits, please fill out the form below. Our lawyers respond to each and every email they receive in a timely manner.
We look forward to hearing from you.