Contents
- 1 Studies Confirm Invokana and Invokamet Linked to Increased Risk of Amputations
- 2 How Common are Diabetes Amputations?
- 3 Why Do People with Diabetes have a Higher Risk of Amputation?
- 4 Risk Factors for Diabetic Amputations
- 5 Diabetic Neuropathy
- 6 Poor Circulation
- 7 Calluses, Ulcers, and Skin Changes
- 8 Invokana Amputations
- 9 Diabetic Amputations vs. Invokana Amputations
- 10 What is Invokana?
- 11 Results of the CANVAS Trial
- 12 Amputation Risk Warnings
- 13 Other Safety Alerts
- 14 The Continuing Problems of Johnson & Johnson
- 15 Filing an Invokana Lawsuit
- 16 Medical Bills and Other Costs
- 17 Pain and Suffering
- 18 Obtain the Help of a Fantastic Invokana Lawyer
Increased Risk of Amputation With Invokana Diabetes Medicine
Based on new data from two large clinical trials, the US Food and Drug Administration (FDA) has confirmed that the Type II diabetes medicine canagliflozin (which has brand names like Invokana, Invokamet XR, and Invokamet) comes with an increased risk of leg, toe and foot amputations.
To describe this risk, the agency added new warnings to canagliflozin drug labels, which includes a prominent “Black Box Warning”.
Studies Confirm Invokana and Invokamet Linked to Increased Risk of Amputations

How Common are Diabetes Amputations?

A serious concern for people with diabetes is amputations. A lot of amputations are performed on the lower extremities, particularly the feet and toes. Although it is less common, some people who have diabetes require amputations below the knee.
Nearly 67% of amputations in the US are associated with diabetes and complications that are related to it. The CDC reports that in 2010, approximately 73,000 non-traumatic amputations of the lower limbs were performed in adults aged 20 years or older who were diagnosed with diabetes.
The rate of amputation for people with diabetes is 28 times higher when compared to those who do have the disease. Amputations are performed more commonly for men than women, with a 50% higher rate of amputation for males with diabetes. Research has also found that the disease affects the non-Hispanic black population and the elderly more frequently.
Why Do People with Diabetes have a Higher Risk of Amputation?

Two major issues that create cause for an amputation are poor circulation and nerve damage, worsened by blood sugar that is left uncontrolled. These complications allow infections to set in without the knowledge of the diabetic, resulting in a gangrenous wound that could require an amputation at a later stage.
Risk Factors for Diabetic Amputations
People with diabetes have sensitive feet with a higher vulnerability to injuries, especially when blood glucose is not managed. All of the factors that cause amputations actually work together, and this is why it is extremely important to be aware of each one.
Diabetic neuropathy reduces sensation and makes patients unaware that they have injuries because they are unable to feel the development of wounds. On the other hand, poor circulation prevents injuries from healing properly. Knowing the risk factors for diabetic amputation plays a key role in prevention.
Diabetic Neuropathy
Diabetic neuropathy is a common complication that comes with diabetes. It is a form of nerve damage that high blood sugar causes. It results in injury to nerve fibers all over the body and leaves patients with a decrease in sensation in the lower limbs and feet.
Neuropathy may prevent people with the disease from feeling hot or cold temperatures. They might not notice a callus or cut, making them more vulnerable to infection.
There are 4 types of diabetic neuropathy: autonomic neuropathy, mononeuropathy, radiculoplexus neuropathy, and peripheral neuropathy.
Symptoms of this complication might include numbness, burning, tingling, and pain. Some people with neuropathy experience vision complications, bladder problems, digestive issues, and erectile dysfunction.
Poor Circulation
Diabetes may cause blood vessels to narrow and harden, limiting the flow of blood to the legs and feet. When blood flow is lacking, it can have an adverse effect on the body’s ability to fight infections and the speed of wound healing. Peripheral vascular and arterial diseases are some identifiable indicators of poor circulation.
Smoking also has an effect on the body’s small blood vessels. It can reduce the flow of blood throughout the limbs make it more difficult for injuries to heal. Many diabetics who need to be amputated are smokers.
Calluses, Ulcers, and Skin Changes
Changes in the skin, related to nerve damage, has an effect on the moisture and natural oils in the feet. When diabetes affects toe and foot skin, it results in dryness, which in turn makes it more likely for the skin to crack and peel. If there are changes in the foot’s natural regulatory state, it makes the patient more vulnerable to infection, injury, and amputation.
Caused by repeated irritation pressure or friction, calluses are also more common with diabetes. Complications can arise from calluses that are not trimmed or treated. They frequently occur on the bottom of the big toe or the ball of the foot.
Calluses may develop into painful open sores called ulcers. Neglecting treatment of ulcers or walking on them can lead to infections that are deeply embedded. Many of these are non-healing wounds that may result in the loss of a limb.
Invokana Amputations

A history of prior amputation may also predispose patients who take Invokana to the need for amputation. Patients with a history of peripheral vascular disease or amputation had the highest risk of amputation, but the relative risk was similar.
Diabetes patients, regardless of a prior risk, are encouraged to discuss the potential amputation side effects of Invokana with their doctors.
Most diabetic foot infections need some type of surgical intervention, but diabetics can actually avoid many foot problems. Taking the time to learn about and understand the risk factors, key elements of proper care and all the risks that come with medications, like Invokana, can make it possible to prevent diabetic foot ulcers and amputations.
The most common amputations that diabetics taking Invokana suffer are toe and middle-of-the-foot amputations. The drug can also cause patients to need amputations of the leg below or above the knee. After taking Invokana, patients may need to have one or both of their limbs amputated.
Diabetics who take Invokana should be closely monitored for signs and symptoms of conditions that might make it necessary to get an amputation performed.
Amputations may be required as a result of infections or ulcers, especially those that reach the bone. It is important to keep in mind that even minor cuts or other punctures or trauma to the skin can result in life-threatening infections. In instances like these, patients may not be able to avoid an amputation.
Patients who take Invokana should inform their healthcare provider immediately if they develop new tenderness or pain, sores or ulcers, and infections in the feet or legs.
Diabetic Amputations vs. Invokana Amputations
According to the American Diabetes Association, diabetics are more likely to have a foot or leg amputated than other people. About 15% of people with diabetes develop ulcers on their foot, and roughly a quarter of those people will require an amputation.
Amputation can be caused by diabetes or the use of Invokana. When the legs and feet do not have proper circulation of blood, it can result in the need for both types of amputation. Invokana amputations begin with the body getting dehydrated because of increased urination that the drug causes. On the other hand, diabetic amputations often begin with blood sugar levels that are poorly controlled.
What is Invokana?
The active ingredient in Invokana, canagliflozin is also one of the active ingredients in Invokamet, which is a combination product and another medication used for treating the disease.
Invokana is one of the few medications that belong to the SGLT2 Inhibitor class of antidiabetics, which includes brand name medications Jardiance and Farxiga, along with combination products Synjardy, Glyxambi, and Xigduo. Since the approval of Invokana in 2013, it has been shown to increase the risk for serious side effects, which include the following:
- Diabetic ketoacidosis
- Kidney disease and renal failure
- Severe urinary tract infection (UTI)
- Increased risk of bone fracture
In May 2016, a safety alert was issued regarding CANVAS and CANVAS-R trials’ interim results which showed the doubling of amputation risk in patients taking Invokana. The risk was confirmed in the trials’ final results, which were reported in 2017.
Based on these final results, the most serious safety alert possible, the Black Box Warning, was issued by the FDA. This warning includes information about the risk of amputation.
Results of the CANVAS Trial
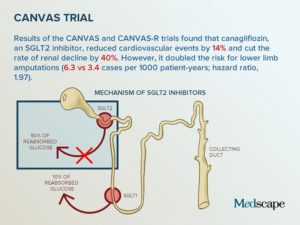
The boxed warning included on Invokana labeling was an addition to the information already printed on the labeling of the drug regarding an increased risk for lactic acidosis due to metformin, one of the ingredients used in Invokana.
As mentioned earlier, the risk of amputation was identified during the large, CANVAS and CANVAS-R studies which were originally undertaken to study the long-term effects of the drug on the heart.
Janssen Pharmaceuticals and Johnson & Johnson, the parent company, had hoped to find positive cardiac results for Invokana but found an increase in the risk for amputation instead.
Patients who participated in the two clinical trials were followed for 5 years. The studies compared patients taking Invokana with those taking a placebo and found that the risk for amputation was as follows:
- The placebo patients experienced amputation at a rate of 3/1,000 patients annually.
- Patients taking a daily dose of 100mg Invokana experienced at a rate of 5/1,000 patients annually.
- Patients taking a daily dose of 300mg Invokana experienced amputation at a rate of 7/1,000 patients annually.
This shows a low dose of Invokana caused an increased risk that is two-thirds higher while a higher dose resulted in more than 2 times the risk of amputation.
Amputation Risk Warnings

Conditions like leg or foot ulcers, neuropathy, peripheral vascular disease and prior history of amputation may add to the increase in the risk of amputation due to inability to sense injury and lack of blood flow. Warnings are issued to practitioners to make sure that they monitor the foot and leg health of patients and discontinue Invokana if there are any complications.
Patients are advised to report any symptoms that affect the health of their skin or extremities, such as tenderness, sores, infection or pain. However, they should not discontinue the medication without first seeking advice from a healthcare professional.
Other Safety Alerts

In March of 2013, Invokana was the first approved SGLT2 inhibitor. By mid-2015, the agency noted that it had received a large number of adverse event reports about the medication and other SGLT2 inhibitors for events involving diabetic ketoacidosis (DKA) which had required hospitalization.
In May of 2015, a safety alert about this development was issued, which was followed by labeling changes in September about bone density loss that contributed to the increase in the risk of fracture.
Another safety warning about DKA was issued in December of 2015, which included warnings about an increase in the risk of severe UTI which may contribute to kidney failure. Updates were made to labeling to include information about DKA, UTI and kidney failure.
As mentioned earlier, the first risk warning about amputation was issued in May of 2016 based on interim results of the CANVAS study.
A month later in June, more information followed to strengthen warnings about kidney injury. The most recent warning – the Black Box Warning – was issued in May of 2017 about the potential of doubled risk of amputation that comes with Invokana and Invokamet.
The Continuing Problems of Johnson & Johnson
Janssen Pharmaceuticals is a subsidiary of Johnson & Johnson, the pharmaceutical giant that has an estimated annual revenue of nearly $72 billion. Due to problems with several of their products that range from surgical products and medical devices to consumer products such as baby powder to a number of drugs that they manufacture, like Invokana, the company has faced a number of medical injury crises.
Injuries caused by Invokana are being evaluated by regulators from across the globe, including the EU where European medical regulators at the Risk Management Committee of the European Medicines Agency have requested Johnson & Johnson to provide more information regarding the amputation risk of Invokana.
Other drugs for treating diabetes, including SGLT2 inhibitors, Jardiance and Farxiga, are also under fire. However, the same warning does not come with either drug as of yet.
Numerous lawsuits involving Invokana have already been filed by patients or family members of those who have suffered serious side effects that have been caused by the drug. Invokana lawsuits have included kidney failure, bone fracture, diabetic ketoacidosis and other serious injuries.
More of these lawsuits may be expected as a result of Invokana causing the need for amputation in some patients who take the medication.
Filing an Invokana Lawsuit

In the complaints included in these lawsuits, plaintiffs say that they would have avoided taking Invokana had they known about the increased amputation risk ahead of time. Since proper warnings were not provided by Janssen Pharmaceuticals, consumers were not able to make an informed choice on the matter.
There have been similar legal complaints about other serious side effects of Invokana. These are related to the various warnings issued by the FDA around renal failure, ketoacidosis, serious urinary tract infections, and bone fractures.
To find out if you are eligible to file an Invokana lawsuit, the first thing you should do is contact an accomplished Invokana amputation lawyer with experience in handling cases involving dangerous drugs.
This will help the lawyer learn more about your situation. Certain factors determine whether you are eligible to participate in an Invokana class action lawsuit or to file an individual lawsuit against the manufacturer. These factors include when you began and stopped taking Invokana, the side effects that it causes, and the severity of the harm you suffered as a result of taking the drug.
If your situation meets the initial criteria for case selection, the lawyer will order, pay for, and review your medical records. Board-certified physicians will also review those records to confirm that Invokana was the cause of your condition. If this can be proven, your legal counselor will determine the option that is best suited for your individual case and they can then immediately pursue that option.
Medical Bills and Other Costs
The reason that many people file Invokana lawsuits is to attempt to obtain compensation to recover costs that are related to medical bills. These costs can include surgery, hospitalization, post-operative care, and ongoing medical needs.
On top of this, the compensation can also cover related costs such as travel expenses incurred to see a specialist, lodging near centers where patients received treatment, and expenses that insurance may not cover.
Pain and Suffering
People who have experienced a severe side effect as a result of taking Invokana claim in their lawsuits that they suffered additional pain and suffering beyond what was expected due to the drug, based on the marketing and warning labels that the manufacturer provided.
While the specific suffering might differ from one patient to another – amputation, ketoacidosis, kidney disease, etc. – the common link between them is that those conditions were exacerbated or caused by Invokana.
In addition to physical pain and suffering that Invokana caused, many patients who took the drug have also suffered mentally. For instance, a number of psychological effects can result from amputation, including phantom limb syndrome, post-traumatic stress disorder (PTSD), and depression. All of these mental health conditions would not have been experienced by patients had they not taken Invokana.
In most cases, Invokana amputation lawsuits include claims to recover these costs that victims of injuries caused by the medication would not have needed to pay otherwise.
For those who passed away due to complications caused by Invokana, their families may ask for compensation to cover funeral and burial costs as well as lost income that their loved one might have otherwise earned.
Obtain the Help of a Fantastic Invokana Lawyer
If you or a loved one has received an amputation or suffered another serious complication or side effect as a result of taking Invokana, you may be eligible to file a lawsuit against the drug manufacturer. Discussing your case with a prudent and motivated Invokana lawyer is the best way to learn about your legal rights and gain more information specific to your case.
It is important to have an Invokana lawyer to help you as you will need help in understanding the strength of your case what to expect if you file an Invokana lawsuit. Fighting back against major drug companies like Johnson & Johnson will require the knowledge, skill, and resources that only an experienced legal pro has.
When you have someone who knows how to fight back against the corporation’s highly trained and well-paid lawyers, you get the best chance of receiving the compensation you deserve for complications you experienced as a result of taking Invokana.

