Contents
- 1 The DePuy Synthes Radial Head Prosthesis Units Taken Off the Market
- 2 The DePuy Synthes Radial Head Prosthesis System Bypassed Strict FDA Review
- 3 DePuy Trial Head Recalls
- 4 Complications Caused by the DePuy Synthes Radial Head Prosthesis System
- 5 Signs of Loosening of the DePuy Synthes Radial Head Prosthesis System
- 6 Depuy Synthes Elbow Lawsuit
What You Need to Know About Depuy Synthes Elbow Replacement – Depuy Synthes, a subsidiary of Johnson & Johnson, developed the Radial Head Prosthesis Device, which is a medically used elbow replacement prosthetic device. This is an elbow joint prosthesis made of two pieces that are used for primary as well as revision joint of the radial head of the elbow.
The DePuy Synthes Radial Head Prosthesis System received clearance from the FDA in June 2011 through the 510(K) program of the agency.
This program does not require manufacturers of devices to conduct human clinical trials if the device-makers can show that a new product is substantially equivalent to a device that the FDA had previously approved.
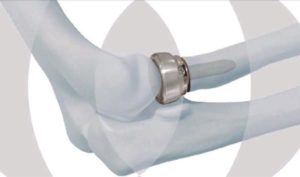
The DePuy Synthes Radial Head Prosthesis System, or elbow replacement system, is a hi-tech modular system that is comprised of cobalt chrome head and titanium alloy stem components, which is combined with an integral side-loading and screw application to allow for in-situ assembly.
There are 24 different heads and 10 different stems, providing surgeons with a choice of 240 potential combinations for the elbow implant. The option of the side-loading application may also allow surgeons to make smaller incisions on their patients.
The DePuy Synthes Radial Head Prosthesis System is indicated for:
- Restoring joint function with primary as well as revision joint replacement of the radial head of the elbow joint
- Replacing the radial head for post-traumatic or degenerative disabilities
- Primary replacement after the radial head has been fractured
- Symptomatic episodes after resection of the radial head
- Revision after unsuccessful arthroplasty of the radial head
The DePuy Synthes Radial Head Prosthesis Units Taken Off the Market

If the device loosens, it may fail to function properly and can result in other health issues, such as pain, post-operative bone fractures, loss of bone tissue, and damage to soft tissues.
As a result, the recall was classified as a Class II medical device recall by the FDA in February 2017. A Class II medical device recall is one that should be taken seriously. The agency class a recall as Class II, when the product may result in a medically reversible or temporary health issue or if there is a slight likelihood that it will cause serious health issues or death.
When a manufacturer recalls one of its medical devices, it basically means that they are acknowledging that there is a potential problem with their product. Reports of adverse events made to the FDA indicate that loosening that occurred with the DePuy Synthes Radial Head Prosthesis System had been reported to the agency for several years prior to the initiation of the recall.
DePuy Synthes has instructed medical professionals to return each and every unused affected product as quickly as they can. The company also instructs health professionals to follow patients who have already received the recalled product as a treatment for their problem in the usual manner.
According to the DePuy-issued “URGENT NOTICE: MEDICAL DEVICE FIELD SAFETY NOTIFICATION”, the removal had an effect on the entire Radial Head Prosthesis System, although it is only the radial stem that can possibly loosen at the stem-bone interface after the surgery is performed.
The company said that based on the data that was currently available, they believed that the cause of the implant’s malfunction was multi-factorial (including possible characteristics of the product as well as patient and operative factors), but they were unable to characterize all of those factors.
As a result, DePuy Synthes was unable to issue any further instructions to doctors and surgeons that might help in reducing the issue rate and made the decision to remove the Radial Head Prosthesis System from the global market.
The DePuy Synthes Radial Head Prosthesis System Bypassed Strict FDA Review

The program is sometimes criticized because it allows companies to skip many of the more formal protocols of the FDA and begin selling their devices sooner than they would if they went through all those protocols.
In June 2011, the DePuy Synthes Radial Head Prosthesis System, as already mentioned, was approved via the 510(K) process. June 2012, saw the merger of Johnson & Johnson’s DePuy franchise and Synthes. However, Synthes had already developed the Radial Head Prosthesis System and guided it through the FDA’s approval process.
Synthesis had presented the elbow implant as substantially equivalent to 12 other devices that were already on the market. The device’s surface, critical for attaching to the bone and potentially important in the loosening of the device, was compared in the application made by the company to 10 other existing medical devices ranging from spinal fusion devices to dental implants to 2 components already sold by Synthes.
It was clearly mentioned in the application by DePuy that their prosthesis system did not in any way raise any new safety or effectiveness issues in comparison to the predicate devices.
The company also said that the elbow implant had the same indications for use, similar materials and the same fundamental technological characteristics as two other radial head devices that were developed and sold by competing medical device-makers.
In its 510(K) application, DePuy Synthes compared their prosthesis system to devices such as:
- Biomet ExploR Modular Head
- 2 different versions Straumann Dental Implant System
- Ascension Modular Head
- 5 versions of Titan Endoskeleton Interbody Fusion Devices
DePuy Trial Head Recalls
In February 2013, DePuy recalled 147 of the DePuy Synthes Radial Head Prosthesis Systems due to an issue with its trial head. A trial head is employed during surgery to ascertain if the actual head that will be implanted will function as intended when after the procedure.
The company is said to have reported a worrisome finding. They claimed that the trial head that was shipped
with the elbow replacement implants could come loose from the stem during surgery. It also said that the problem did not cause any adverse events at the time of the earlier recall.
Complications Caused by the DePuy Synthes Radial Head Prosthesis System

The natural radial head is removed during surgery, and the stem is inserted into the bone’s end. The implant is designed to “press-fit” into the bone. The stem’s surface is roughened in order to promote the growth of the bone around the stem and hold it securely in place.
If the implant loosens, it can result in problems in the way that the implant functions and can cause other health issues.
If the implant loosens, the problem may be corrected only through revision surgery to replace or repair the device. In DePuy Synthes’ product removal notice, the company told health care professionals, as mentioned earlier, to continue to follow patients who have already received the implant in the usual manner.
There have been reports of complications reported by patients who have received a DePuy Synthes elbow implant. These complications include:
- Corrective, or revision, surgery due to complications resulting from the loosening of the elbow implant
- Symptomatic loosening implant that has not yet been resolved through surgery
- Pain
- Osteolysis, or bone loss
- Impaired range of arm and joint motion/poor joint mechanics
- Fracture of the bone around the implant as a result of loosening
- Damage to or aggravation of soft tissue
Signs of Loosening of the DePuy Synthes Radial Head Prosthesis System
Not all stems of the DePuy Synthes Radial Head Prosthesis System loosen and determining if any individual implant will fail can be difficult. But the first sign of a problem may be pain in the forearm.
A study that was printed in the Journal of Shoulder and Elbow Surgery in 2012 warned readers that aches and pain that occurs after a radial head replacement may often be misinterpreted and that there is a lack of reliable guidelines for diagnosing loosening of the radial head prosthesis.
The authors of the study also pointed to pain the forearm as a major indication of loosening of implants that are similar to the design of the DePuy elbow implant.
According to the researchers, when a patient with a radial head prosthesis suffers from proximal radial forearm pain, it is an indication of symptomatic mechanical loosening. If the prosthetic unit has a textured surface to support the ingrowths of the bone and was implanted without the use of cement, it is considered a strong indicator for loosening, even when there are no radiographic signs.
Signs that indicate loosening of an elbow replacement implant include pain, swelling, and impaired range of motion. If a patient who has had surgery for elbow replacement experiences these issues, a doctor will rely on certain tests to determine if the actual cause is loosening of the implant. They may order one or more
examinations like:
- Bone scans
- X-rays
- Other diagnostic imaging or clinical work-ups
Depuy Synthes Elbow Lawsuit

By filing a Depuy Elbow Lawsuit, you may be able to obtain financial compensation for your injuries. Fill out the form below to contact our Patient Advocates handling these claims.